Section 1: Body Chemistry
LEARNING OBJECTIVES
After reading this section you should be able to:
- Differentiate between atoms, ions and molecules.
- Differentiate between elements and compounds.
- Differentiate between acids, bases, and electrolytes.
- State the significance of the pH scale.
- Explain the importance of acid-base balance in the body.
- Explain the terms metabolism, anabolism and catabolism.
- Describe the structure and state the function of carbohydrates, proteins, lipids and nucleic acids.
- State the function of enzymes, substrates, cofactors and coenzymes.
- Explain how enzyme-mediated reactions are regulated.
ATOMS AND IONS
Just as the cell is the basic unit of living things, the atom is the fundamental unit of matter.
| Did you know?
Atoms are very small particles – millions of atoms would fit on a pinhead. |
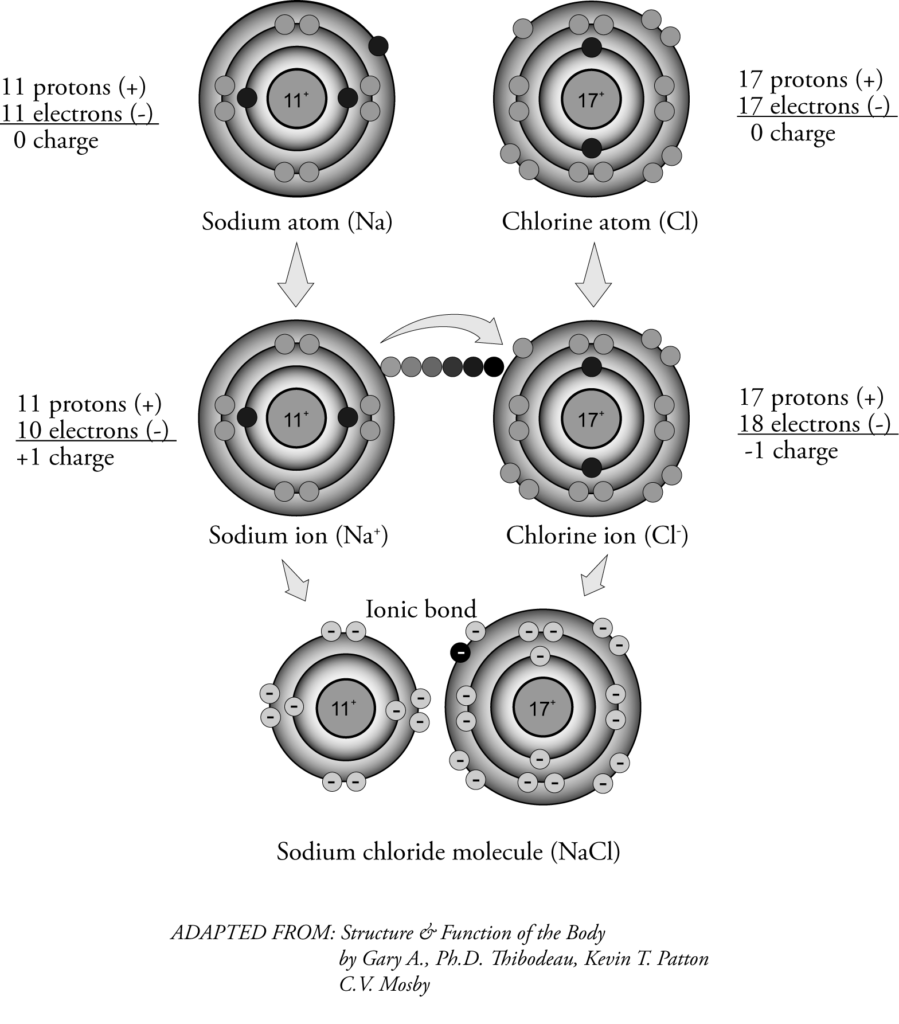
A nucleus in the centre of an atom contains protons, which are positively charged electric particles, and neutrons, which are noncharged particles. Electrons, which are negatively changed particles, surround the nucleus in orbitals or shells. (See Figure 1.1‑1.) Because the number of positively charged protons and negatively charged electrons is equal, the atom does not have an overall positive or negative charge.
Atoms that have 8 electrons in their outermost orbital are stable. Metal atoms have 1, 2 or 3 electrons in their outermost shell. Non-metals have 5, 6 or 7 electrons in their outer shell. Inert gases, such as radon, have 8 electrons and are stable, which is why they are called inert.
To become stable, the metal ion must get rid of the electrons in its outer shell. The non-metal ion can receive the electrons from the metal ion, thus making the outer shell of both the metal atom and non-metal atom stable.
If the atom gains an electron, it becomes negatively charged. If the atom loses an electron, it becomes positively charged. An atom with a positive or negative charge is called an ion*. Cations are positively charged ions. Anions are negatively charged ions.
Figure 1.1-1 shows a sodium atom coming in contact with a chlorine atom. The chlorine atom gains an electron from the sodium atom, and the two new ions (Na+ and Cl–) attract each other due to their opposite charges. The ions cling together and produce the compound* sodium chloride. The bond formed by the transfer of electrons is called an ionic bond.
Figure1.1-1: Atom and Ion
MOLECULES
A molecule* is formed when two or more atoms unite. A molecule may contain identical atoms or two or more different atoms. For example, an oxygen molecule (O2) contains two oxygen atoms while a molecule of water (H2O) contains two hydrogen atoms and one oxygen atom.
ELEMENTS AND COMPOUNDS
Elements* contain only one kind of atom. They cannot be broken down into substances different from themselves. There are approximately 105 known elements.
The elements that make up most of the cells in the body are:
- carbon (C)
- hydrogen (H)
- oxygen (O)
- nitrogen (N)
Other important elements in the body are:
- iron (Fe)
- potassium (K)
- calcium (Ca)
- sodium (Na)
- phosphorus (P)
- sulfur (S)
- chlorine (Cl)
- magnesium (Mg)
Compounds are substances that contain two or more different elements. Water (H2O) is an example of a compound.
ACIDS AND BASES
An acid* is a chemical substance that can donate a hydrogen ion (H+) to another substance. An example of an acid is hydrochloric acid, the acid that is found in the stomach. The box below illustrates the equilibrium that exists in solution between the undissociated acid on the left and its dissociation products on the right.
| HCI
Hydrochloric acid |
<-> | H+
Hydrogen ion |
+ | CI–
Chlorine ion |
A base* is a chemical substance that can accept a hydrogen ion (H+) from another substance. Bases usually contain the hydroxide ion (OH-). An example of a base is sodium hydroxide. The box below illustrates the equilibrium that exists in solution between the undissociated base on the left and its dissociation products on the right.
| NaOH
Sodium hydroxide |
<-> | Na+
Sodium ion |
+ | OH–
Hydroxide ion |
ELECTROLYTES
Substances that dissociate in water into cations (positively charged ions) and anions (negatively charged ions) are classified as electrolytes* because these ions facilitate conductance of an electric current through water.
- strong electrolytes – Sodium (Na) and potassium (K) salts, such as sodium chloride (NaCl) and potassium chloride (KCl), are good examples. In solution, NaCl dissociates completely into Na+ and Cl– ions, which are called strong electrolytes.
- weak electrolytes – Many drugs are either acids or bases. When they are dissolved in water, they dissociate into ions that act as electrolytes. However, many acids and bases when dissolved in water do not dissociate totally. Instead, they establish equilibrium between undissociated and dissociated components. Although anions and cations exist in solution, they have a lower capacity to carry an electrical charge and are termed weak electrolytes.
- nonelectrolytes – Sugars and alcohols are classified as nonelectrolytes because they dissolve readily in water, but do not carry a charge or dissociate into charged particles.
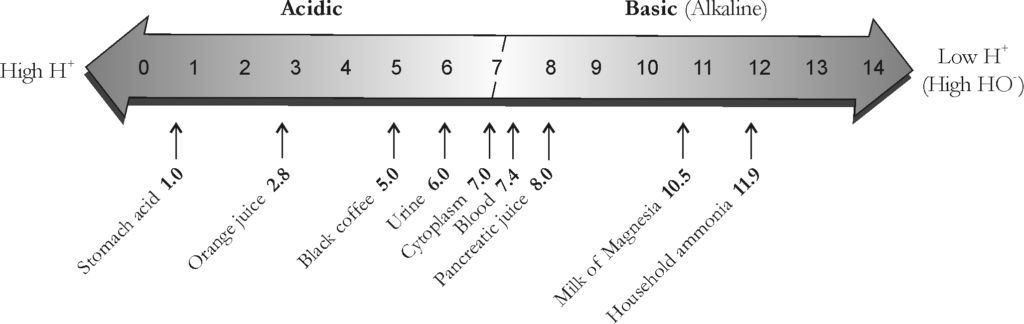
PH SCALE
The pH scale* measures the concentration of hydrogen ions in a solution. The scale ranges from 0 to 14. Each descending pH unit on the scale equals a tenfold increase in the number of hydrogen ions present. For example, a solution with a pH of 4.0 has ten times the number of hydrogen ions as a solution with a pH of 5.0.
The greater the concentration of hydrogen ions in a solution, the more acidic* the solution becomes. The most acidic solution would have a pH of 0. The lower the concentration of hydrogen ions in a solution, the more alkaline* (basic*) the solution becomes. The most basic solution would have a pH of 14.
Therefore, the lower the pH, the more acidic is the solution, and the higher the pH, the greater is the alkalinity. (See Figure 1.1-2.) For example, stomach acid has a pH of 1.0, whereas blood is slightly alkaline with a pH of 7.4.
A solution with an equal number of hydrogen and hydroxide ions is said to be neutral and has a pH of 7.0. Pure water and cytoplasm are examples of substances with a neutral pH.
Figure 1.1-2: The pH Scale
ACID-BASE BALANCE IN THE BODY
Acids and bases are compounds that affect chemical reactions in the body. A delicate balance exists in the acidity or alkalinity of body fluids. For example, blood is slightly alkaline, urine is slightly acidic, the stomach juices are more acidic, and the intestinal and pancreatic juices are alkaline.
The acid-based balance must be maintained in the body because:
- a change in hydrogen ion concentration (above or below normal) will disrupt normal chemical reactions in the body.
- pH affects movement of electrolytes across cell membranes and can have a pronounced effect on various body functions.
Tissue damage can occur if tissues are exposed to pH conditions that are outside of the normal range for those tissues. For example, if an abnormally high amount of acid is secreted in the stomach or mucosal defense of the gastrointestinal (GI) tract is impaired, the lining of the GI tract may be injured due to exposure to stomach acid.
In blood, carbon dioxide (CO2) forms carbonic acid (H2CO3) when it dissolves in water. Some of the carbonic acid then dissociates to form H+ ions and HCO3– (bicarbonate) ions, producing an excess of H+ ions in the blood. Therefore, high levels of CO2 in the blood make blood more acidic (acidosis*). If there is too little carbon dioxide in the blood, then the blood becomes more alkaline (alkalosis*). The bicarbonate ion concentration and the pH of blood samples are measured to assess a patient’s acid-base status. Normally, the lungs and the kidneys work together to regulate the acid-base balance in the blood by maintaining pH and carbon dioxide levels within strict limits. The lungs regulate pH by controlling the amount of CO2 in the blood (i.e., lungs eliminate CO2 in expired air). The kidneys regulate HCO3–, the base component of pH (i.e., kidneys reabsorb all of the bicarbonate that is filtered out of the blood). The role of the lungs in acid-base balance is discussed in more detail in Module 5, Section 2, and the role of the kidneys in Module 7, Section 2.
METABOLISM
Many physical and chemical reactions take place in the cells of the body to sustain life. These cellular reactions are referred to as metabolism*.
Metabolism involves two types of reactions:
- catabolism*
- anabolism*
Catabolism
Catabolism is destructive metabolism, where large complex substances are broken down into simpler smaller substances, with the release of energy. An example of catabolism is the break down of stored nutrients such as glycogen* and fat into smaller units that can be used as energy by cells of the body.
Anabolism
In anabolism, simple compounds are converted to complex substances required for cellular activities, such as the growth, functioning and repair of cells. It also includes the storage of energy. An example of anabolism is the conversion of glucose into glycogen.
ORGANIC COMPOUNDS
Organic compounds* contain the element carbon.
Carbon is able to bond to many different elements and many carbon atoms can join together to form long chains. Therefore, most organic compounds are large, complex molecules.
There are four main types of organic compounds:
- carbohydrates*
- proteins*
- lipids*
- nucleic acids*
We’ll look at each of these organic compounds in more detail. Each of these organic compounds is made up of subunits, which act as building blocks for the compound.
Carbohydrates
Carbohydrates contain carbon, hydrogen, and oxygen.
Monosaccharides*, which are simple sugars, are the building blocks of carbohydrates. When monosaccharides join together, they form polysaccharides*, which are complex carbohydrates.
Carbohydrates are the principal source of energy in the body. Glucose is a simple sugar found in blood and is a readily usable source of energy for cells. Glucose is stored as starch in plants and as glycogen in animals. Starch and glycogen are polysaccharides made up of many glucose units.
Proteins
Proteins contain the element nitrogen in addition to carbon, hydrogen, and oxygen. Some proteins may also contain sulphur, phosphorus and iron. The building blocks of proteins, amino acids*, join together to form a long chain. The number, kind and sequence of the amino acids in the chain determine the primary structure of a protein. The long chain may fold back and forth on itself, forming a globular molecule. Each protein has a specific spacial configuration, which has led to development of targeted therapies.
Proteins are the structural materials of the body. They are found in bone, muscle and connective tissue, and are essential for growth, building and repairing of tissue. Pigments found in hair, eyes, and skin also contains proteins.
Lipids
Lipids are another type of organic compound. The most commonly known lipids are fats and fat-related substances that are used by cells for long-term energy storage. They also insulate and protect body organs. The building blocks of fats are glycerol* and fatty acids*.
There are several important types of lipids in the body:
triglycerides* – Triglycerides are lipid molecules formed by a glycerol unit joined to three fatty acids. Their bonds can be broken to yield energy. Triglycerides store energy in cells for later use.
phospholipids* – Phospholipids are similar to triglycerides but one fatty acid has been replaced by a phosphate group (PO4). Phospholipids are made up of a hydrophilic* head (phosphate group) and a hydrophobic* tail (two fatty acid groups). This structure enables them to form a stable bilayer* in water that forms the foundation for the cell membrane.
steroids* – Cholesterol* is an important steroid. It combines with phospholipids in cell membranes to stabilize its bilayer structure. Cholesterol is used in the production of steroid hormones* such as estrogen*, testosterone* and cortisone*.
Nucleic acids
Two forms of nucleic acid are found in cells: deoxyribonucleic acid (DNA)* and ribonucleic acid (DNA)*
-
- deoxyribonucleic acid (DNA)
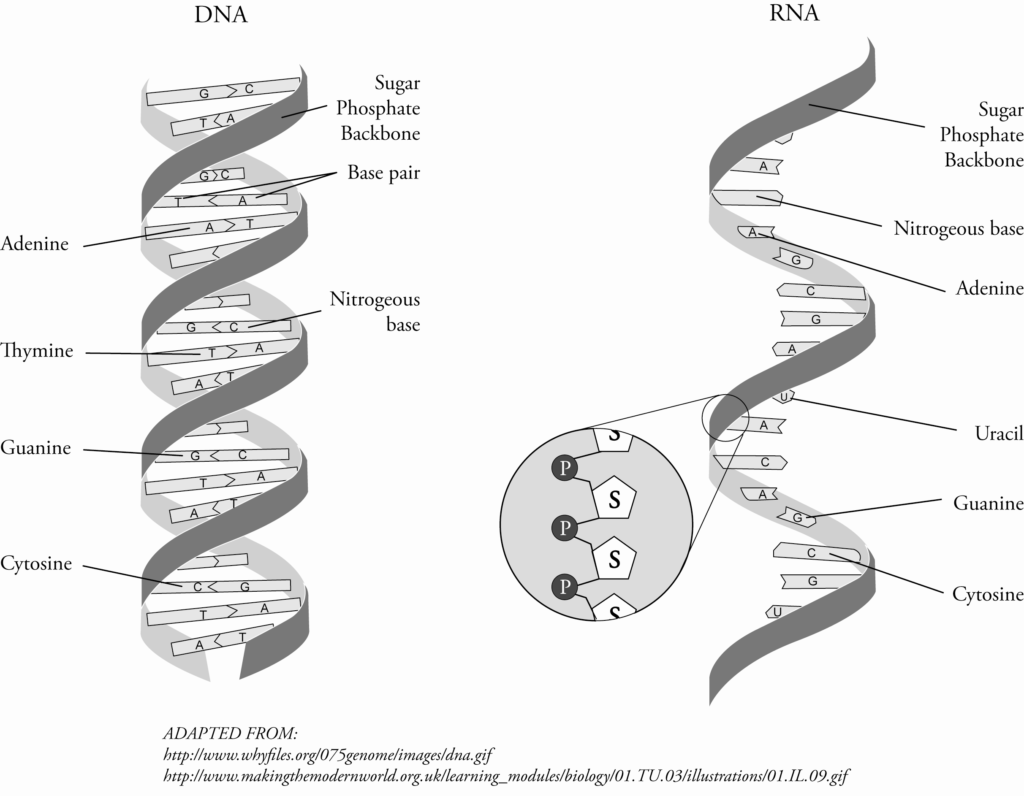
Deoxyribonucleic acid (DNA) molecules are very large molecules made up of two strands that are bonded together and shaped like a double helix. (See Figures 1.1-3 and 1.1-4.)
Each strand is made up of repeating units known as nucleotides*. Each nucleotide contains a phosphate group, the sugar deoxyribose, and a nitrogen base. There are four types of bases: adenine (A), guanine (G), cytosine (C), and thymine (T). Due to their chemical make-up, adenine bonds to thymine and guanine bonds to cytosine. These bonds hold the two DNA strands together.
The number, type, and arrangement of the bases of DNA determine what kind of proteins the cell can form. The importance of DNA in determining the genetic make-up of cells is discussed in more detail in Section 2.
-
- ribonucleic acid (RNA)
Ribonucleic acid (RNA) is similar to DNA in structure except that it is made up of only a single strand.
RNA contains the base uracil (U) rather than thymine, and the sugar ribose rather than deoxyribose. Uracil can bind to adenine.
RNA is also required for the formation of proteins in the cell. The role of RNA in protein synthesis is discussed in more detail in Section 2.
Table 1.1-1: Organic Compounds
| Organic Compound | Subunit or Building Block |
| Carbohydrate | monosaccharide |
| Protein | amino acid |
| Lipid | glycerol and fatty acids |
| Nucleic acid | nucleotide |
Figure 1.1-3: DNA Nucleotides
(4 nitrogen bases are illustrated)
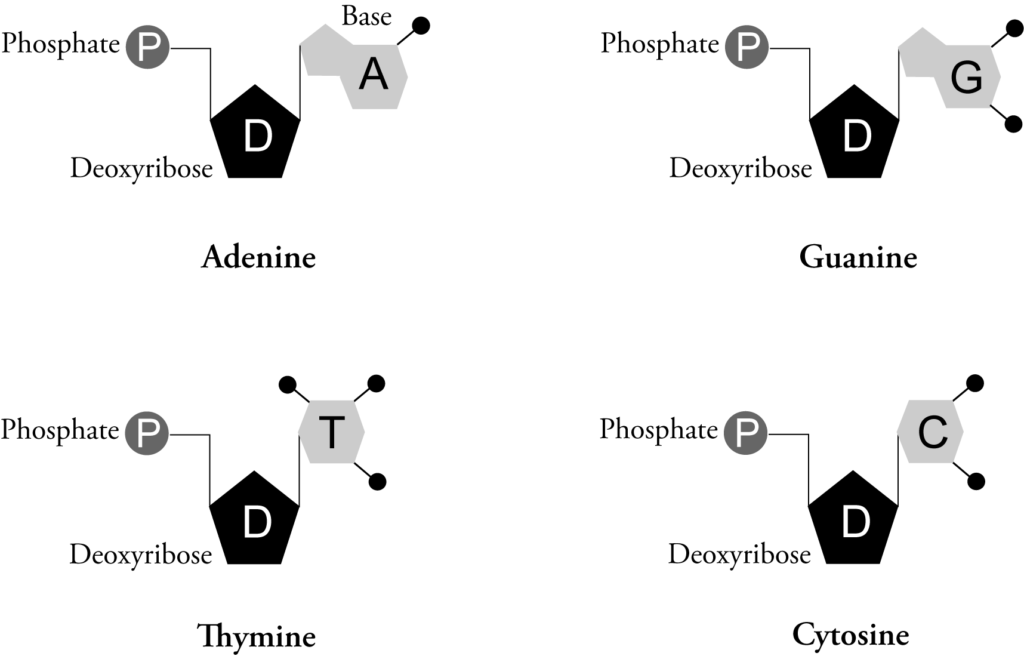
Figure 1.1-4: Structure of DNA and RNA
ENZYMES
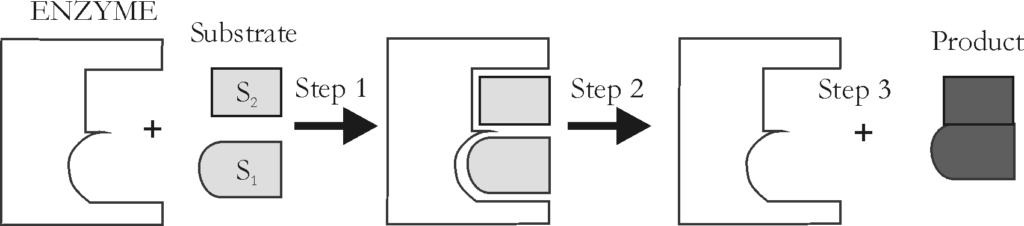
Enzymes* are one important group of proteins. Enzymes are organic catalysts that speed up the rate of a metabolic reaction, but are not changed or used up in the reaction. For each chemical reaction in the body, there is a specific enzyme involved.
A substrate* requires an enzyme in order to be converted to a new product. When the appropriate enzyme is present, substrates are able to line up at the active sites on the enzyme, react, and form a new product. (See Figure 1.1-5.)
Sometimes, an enzyme requires the presence of a cofactor in order to function properly. Examples of cofactors are trace metals such as iron, magnesium or calcium. A coenzyme is a cofactor that is an organic substance.
Figure 1.1-5: Enzyme Action
FACTORS AFFECTING ENZYME-MEDIATED REACTIONS
The rate of an enzyme-mediated reaction can be affected by the following factors:
- substrate concentration
- enzyme concentration
- enzyme activity
- Substrate concentrationA change in the amount of substrate in a cell will affect the rate of the metabolic reaction. An increase in the amount of substrate will result in an increase in the rate of reaction up to the point where all the active sites on the enzyme are occupied (i.e., the enzyme is saturated) by the substrate. Once this maximum rate is reached, the rate of reaction will remain constant no matter how much more substrate is present.
- Enzyme concentration
An increase in the amount of enzyme that is available will result in an increase in the rate of an enzyme-mediated reaction because more active sites are available for the substrate to attach to.
- Enzyme activity
The activity of an enzyme can be affected by a number of factors. An enzyme can be suppressed from catalyzing its normal metabolic reaction by introducing a competing substrate, which binds to the enzyme in place of the normal substrate. If a substance is present that has the same shape as one of the normal substrates, it can occupy the active site on the enzyme and inhibit the activity of the enzyme on the normal substrate.Extreme temperatures or changes in acidity can also alter the shape of an enzyme, which decreases its activity.
SUMMARY — SECTION 1: BODY CHEMISTRY
All matter, whether living or non-living, is composed of chemical elements and compounds:
- atom – basic unit of matter containing protons, electrons and neutrons
- molecule – a combination of two or more atoms that may or may not be identical
- element – composed of one kind of atom
- compound – composed of two or more different elements
Ionic bonding occurs when atoms or groups of atoms are attracted to each other and bind together through the exchange of electrons. Because electrons have a negative charge, addition of electrons produces a negative charge. Loss of electrons produces a positive charge. Ions in an ionic compound are held together by the attraction of these opposite charges. When heated or dissolved in solution, ionic compounds will dissociate and conduct electricity, and are called electrolytes. Compounds that do not dissociate totally in solution (including salts and acids) have a low capacity to carry an electrical charge, and are called weak electrolytes.
Acids and bases are compounds that are involved in chemical reactions in the body. The degree of acidity is measured by pH. The pH of various body fluids can vary from high acidity in the stomach to low acidity (i.e., higher in alkalinity) in the intestines. The acid-base balance of blood is maintained in a strict range through the combined actions of the lungs and the kidneys, which compensate for alterations in carbon dioxide (CO2) or pH.
Metabolism refers to the physical and chemical reactions that occur in the body to sustain life. Anabolism is the cellular building up (synthesis) of organic molecules or storage of energy. Catabolism is the cellular breakdown of organic molecules or release of energy for use by cells.
Four main types of organic compounds make up the structure of the body. These compounds and their subunits are:
- carbohydrates – monosaccharide’s (simple sugars) and polysaccharides
- proteins – amino acids
- lipids – glycerol and fatty acids
- nucleic acids (DNA and RNA) – nucleotides
Enzymes are proteins that influence the speed at which metabolic reactions occur. Factors that affect enzyme-mediated reactions include:
- substrate concentration
- enzyme concentration
- enzyme activity
PROGRESS CHECK — SECTION 1: BODY CHEMISTRY
- Match the description in Column A with the correct term in Column B.
|
Column A |
Column B |
|
| 1) positively charged atom | _____ | a. proton |
| 2) negatively charged particle surrounding nucleus of atom | _____ | b. neutron |
| 3) substance containing only one kind of atom | _____ | c. electron |
| 4) positively charged particle in nucleus of atom | _____ | d. ion |
| 5) ions that conduct electricity when in solution | _____ | e. cation |
| 6) substance with pH of 3.0 | _____ | f. anion |
| g. element | ||
| h. molecule | ||
| i. compound | ||
| j. electrolyte | ||
| k. acid | ||
| l. base |
2. The greater the concentration of hydrogen ions in a solution, the more acidic the solution becomes.
a) true
b) false
3. Why is maintenance of the acid-base balance in body fluids critical to maintaining a healthy state?
4. Define the following terms:
1) Metabolism
2) Catabolism
3) Anabolism
5. For each of the main organic compounds, state the building block or subunit.
carbohydrates
proteins
lipids
nucleic acids
6. Cholesterol is an important component of _ and _
7. Compare the structure of DNA and RNA by completing the following chart.
|
Composition |
DNA |
RNA |
| Sugar | ||
| Bases |
|
|
| Number of strands |
8. State the function of enzymes in a cell.
9. List 3 factors that affect enzyme-mediated reactions in cells.
1)
2)
3)
10. List 3 ways in which enzyme activity might be disrupted.
1)
2)
3)
PROGRESS CHECK ANSWERS — SECTION 1: BODY CHEMISTRY
1.
1 e. cation
2 c. electron
3 g. element
4 a. proton
5 j. electrolyte
6 k. acid
2. a) true
3. If the pH of body fluids is altered, normal chemical reactions and body functions are disrupted, which may damage tissues.
4.
1 Metabolism – physical and chemical reactions that take place in the body to sustain life.
2 Catabolism – breaking down of large complex substances into simpler, smaller substances with the release of energy for use by cells.
3 Anabolism – building up of larger, more complex substances and storage of energy.
5.
carbohydrates monosaccharides
proteins amino acids
lipids glycerol and fatty acids
nucleic acids nucleotides
6.
steroid hormones (e.g., estrogens, testosterone, cortisone)
cell membranes
7.
| Composition | DNA | RNA |
| Sugar | deoxyribose | ribose |
| Bases | adenine
thymine guanine cytosine |
adenine
uracil guanine cytosine |
| Number of strands | 2 (in a double helix formation) | 1 |
8. Enzymes are organic catalysts that speed up the rate of reactions in cells but are not changed or used up in the reactions.
9.
1 substrate concentration
2 enzyme concentration
3 enzyme activity
10.
1 use of a competing substrate
2 extreme temperatures
3 changes in the acidity of the cell
