Who Dunn it? Really?
On Sept 3, 2019, the British Broadcasting Corporation (BBC) reported on-line, the results from a Canadian led study published in the Lancet that headlined: ‘Cancer- biggest middle-age killer in rich nations’ ” and “…now causes more deaths among the middle-aged in higher-income countries than cardiovascular disease…”
Almost 20 years before, cancer researchers Douglas Hanahan of the University of California and Robert Weinberg of the Massachusetts Institute of Technology published their review The Hallmarks of Cancer in the distinguished and peer reviewed journal CELL. By November of 2010, it had been referenced over 15 000 times by other research papers and was accessed for download 20 000 times per year between 2004 and 2007. Academics’ way at ‘going viral’!
For more on the Hallmarks of Cancer and other topics; Go to the Rolling Reference for this Chapter

Though updated in 2011 to include four more characteristics (to be covered later in this chapter); what made this original document so important to medicine was that for the first time, researchers had presented and argued the scientific facts for the foundation of six common traits of cancer; including:
- “Cancer cells stimulate their own growth (self-sufficiency in growth signals).”
- “They resist inhibitory signals that might otherwise stop their growth (insensitivity to anti-growth signals).”
- “They resist their programmed cell death (evading apoptosis).”
- “They can multiply indefinitely (limitless replicative potential).”
- “They stimulate the growth of blood vessels to supply nutrients to tumours (sustained angiogenesis).”
- They invade local tissue and spread to distant sites (tissue invasion and metastasis).”
So you know:
“Survival factors bind to cell-surface receptors and activate signaling pathways that suppress apoptosis.”“ ‘It is usually not a primary cancer tumour that causes patient death’, said Benjamin Martin, PhD, Associate Professor in the Department of Biochemistry and Cell Biology, Stony Brook University School of Medicine in New York. ‘Growth of a primary tumour is not as concerning as cells that leave the primary tumour and form metastatic tumours in other parts of the body,’ Martin noted. ‘A primary tumour can be treated with drugs, radiation therapy, or cut out. But when cells leave that tumour, spread to other parts of the body, and become new tumours, it’s a losing battle over time. The spread of cancer is what kills patients.’”
“The spread of cells is an intense area of research… Martin is hopeful of eventually finding genes capable of keeping cells in a non-dividing state… with the eventual goal of identifying new pathways where we can arrest a cell from dividing and treat it with another drug that would prevent it from being able to invade. In other words, the long-term goal is to be able to eliminate both the growth and spread of the cancer.”
“For thousands of years, questions about the origins of cancer and how to treat the disease have produced few good answers”. “In the last century there have been moderate improvements in treating cancer, and in the last decade or two, researchers have come to a unifying overview of how cancers arise.” As of 2017, world-wide, “Lung and breast cancer are the leading causes of death, followed by colorectal, prostate, liver, stomach, and cervical cancers.”
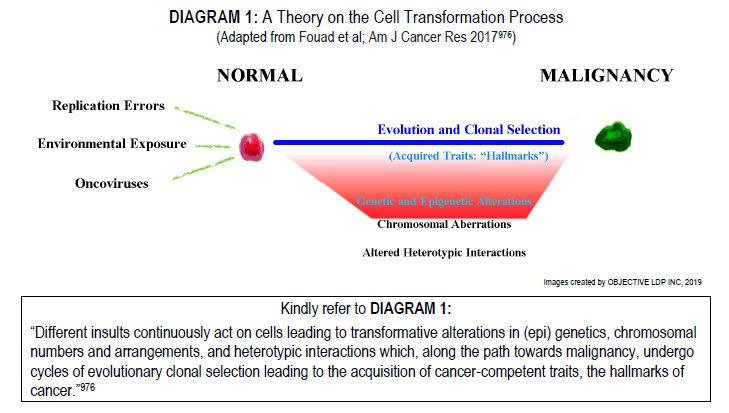
Perhaps a ‘big picture’ mindset is best taken by one who seeks a better understanding of what cancer is. Since, like mutating tumours themselves, the knowledge relating to these diseases is just as protean; transforming and changing routinely so that core principles once accepted as valid for decades; such as the theories surrounding stemness, clonality, the monoclonal origin of tumours and their related tests like X-chromosome inactivation assaying (HUMARA Test) are now all being reconsidered. Even the hallmarks of cancer is up for debate,976 as well as related topics that include whether the methylation of DNA on tumour suppressor genes in colorectal cancer is truly ‘epigenetic’ or not.
Cancer is not a single disease, and it should be noted well that there are over 200 different types of human cancer. Some main-line kinds may be named anatomically – according to the organ involved (eg. breast, lung, bone, paranasal sinus etc.) or histologically – for the type of cell tissue out of which it arises (eg. carcinoma, sarcoma, etc.) or as it specifically relates to its original ‘discoverer’ (eg. Hodgkin, Kaposi, Ewing); perhaps, another disease process all together – like AIDS-related cancers or even others; more rare, like those associated with perturbation-such as the case with Breast implant–associated anaplastic large-cell lymphoma [BIA-ALCL].
“Until recent years, the principal focus in cancer research has mostly been the malignant cell itself. As a consequence, today, there is a significant discrepancy between the vast knowledge about cancer biology generated in experimental settings and the translation of this knowledge into information that can be used in clinical decision making. Understanding the nature of the tumour environment today may be equally important for future cancer therapies as understanding cancer genetics per se. Cancers are not simply autonomous neoplastic cells but [are] also composed of fibroblasts, immune cells, endothelial cells, and specialized mesenchymal cells. These different cell types in the stromal environment can be recruited by malignant cells to support tumour growth and facilitate metastatic dissemination.” For example, the “ECM [Extracellular Matrix] is a major component of tumour microenvironment [TME] and plays critical roles in cancer development and progression.” requiring “extensive reorganization of extracellular matrix.” so that “Increased ECM proteins deposition and crosslink provide necessary biochemical and biophysical cues to promote cancer cell proliferation, migration, and invasion.” “The TME could be depicted as a smoldering site of inflammation where a large number of infiltrated or resident cells produce and release cytokines, chemokines, and enzymes…”
The “Extracellular matrix (ECM) is a complex mixture of structural proteins, glycoproteins, and proteoglycans, which provide not only essential physical scaffolds to maintain tissue structure but also various biochemical signals to modulate cellular function.” For instance, “Altering the fine balance of ECM signal is sufficient in the long run to induce breast cancer development and progression. Increased deposition of collagen and other ECM molecules enhances the cancer tissue stiffness.” “Cellular responses are tissue and context dependent in terms of both biochemical and biomechanical cues (Bissell and Radisky, 2001; Yu et al., 2010). Hence, understanding the complex processes surrounding ECM production, modification and remodeling, and relating these processes to physiological changes in the biochemical and biomechanical properties of the ECM, are key to determining how microenvironmental changes influence cellular responses. These considerations are especially important in the development of antifibrotic and anti-cancer therapies, which might be able to target aspects that are dysregulated in both types of disease.”
“Cells in most tissues interact with an elastic microenvironment that provides not only chemical signals but also inputs of a physical nature. The mechanical properties of a cell’s microenvironment can play a significant role in governing cellular behaviors.”
According to the Sol Goldman Pancreatic Cancer Research Center at Johns Hopkins University, fundamentally, “cancers are often very irregularly shaped, and … like a crab, they ‘grab on and don’t let go.’ The term cancer specifically refers to a new growth [or neoplasm] which has the ability to invade surrounding tissues, metastasize…[and] which may eventually lead to the patient’s death if untreated.” Neoplasms exist as a new growth of cells for which the normal control of cell proliferation, maturation and where normal cell death (apoptosis) is lost. The terms neoplasm and tumour are sometimes used interchangeably. But, “the terms tumour and cancer are sometimes [also] used interchangeably which can be misleading. A tumour is not necessarily a cancer. The word tumour simply refers to a mass… A cancer is a particularly threatening type of tumour. It is helpful to keep these distinctions clear…”
And, “…although physicians know very well what they mean when they use the term [tumour], the question [of what it is] is not a simple one to answer in a concise and comprehensive manner. The word [TUMOUR] ‘tumor’ is of Latin origin and means ‘swelling’ [ONKOS (ὄγκος) in Greek means mass/bulk; as in oncogene]. But not all swellings (eg, the swellings of inflammation and repair) are tumours in the modern sense of the term.”
“The distinguished pathologist Wallace H. Clark has offered an excellent definition, paraphrased as follows: a tumour (fully evolved) is a population of abnormal cells characterized by temporally unrestricted growth and the ability to grow in at least three different tissue compartments—the original compartment; the mesenchyme of the primary site (tumour invasion); and a distant mesenchyme (tumour metastasis). This definition usefully emphasizes the progressive nature of tumour growth, the common (though not exclusive) origin of tumours as benign growths, their gradual acquisition of autonomy, and, at some stage, their ability to grow in new tissues distant from their site of origin, that is, to metastasize.” Based on their cell characteristics and their ability to spread, tumours can be classified as benign or malignant. A malignant neoplasm is a malignant growth and is therefore a ‘cancer’. The term ‘Malignant’ comes from the Latin word MALIGNUS, and means malicious. Malignant neoplasms grow rapidly and are characterized by local invasiveness. Areas of necrosis seen in some malignant neoplasms presumably result when growth is so rapid that the neoplastic tissue outgrows the existing blood supply. Malignant growth is disorganized, and such neoplasms may spread by extension into adjacent tissues or by metastasis to distant sites via blood and/or lymphatic circulation.”
So you know:
“…cancer evolves as the result of an accumulation of mutations within a single cell. Cells have enzymes whose function is to repair defects in DNA. The genes that encode for these enzyme repair mechanism [DNA repair Enzymes] can also be damaged by mutations. In addition, there are several inherited defects in DNA repair that increase the likelihood of transition to malignancy. For example, xeroderma pigmentosum is an inherited defect in DNA repair that causes extreme sensitivity to ultraviolet light, making them extremely vulnerable to sunburn and skin cancers. Ultraviolet light penetrates the superficial layers of the skin, and causes damage to DNA. when the radiant energy is absorbed.”
“In general, the cancer must reach a size of one centimeter (that is, between one-third and one-half of an inch), or be comprised of one million cells, before it is detected. Exceptions to this general rule include cancers of the blood and bone marrow – called lymphomas and leukemias – which frequently do not produce a ‘mass,’ but will be evident on laboratory tests; these cancers still require more than a million cells to be present before they are detected. Lymphomas and leukemias are examples of ‘liquid tumours’ or cancers present in body fluids (the blood and bone marrow), and are detectable by blood laboratory tests.”
So you know:
“While the first observations on metabolic alterations that are characteristic for tumours were first made nearly a century ago, the field of cancer metabolism has become a topic of a renewed interest in the past decade. Aided by new biochemical and molecular biological tools, studies in cancer cell metabolism have expanded our understanding of the mechanisms and functional consequences of tumour-associated metabolic alterations at various stages of tumourigenesis. In particular, it has become evident that tumourigenesis-associated metabolic alterations encompass all stages of cell-metabolite interaction, (a) affecting the metabolite influx through conferring an increased ability to acquire the necessary nutrients; (b) shaping the way the nutrients are preferentially assigned to metabolic pathways that contribute to cellular tumourigenic properties, as well as (c) exerting long-ranging effects on cellular fate, among which are alterations in differentiation of cancer cells themselves as well as of the components of the tumour microenvironment”“Cancer cells exhibit a dynamic metabolic landscape and require a sufficient supply of nucleotides and other macromolecules to grow and proliferate. To meet the metabolic requirements for cell growth, cancer cells must stimulate de novo nucleotide synthesis to obtain adequate nucleotide pools to support nucleic acid and protein synthesis along with energy preservation, signaling activity, glycosylation mechanisms, and cytoskeletal function. Both oncogenes and tumour suppressors have recently been identified as key molecular determinants for de novo nucleotide synthesis that contribute to the maintenance of homeostasis and the proliferation of cancer cells. Inactivation of tumour suppressors such as TP53 and LKB1 and hyperactivation of the mTOR pathway and of oncogenes such as MYC, RAS, and AKT have been shown to fuel nucleotide synthesis in tumour cells. The molecular mechanisms by which these signaling hubs influence metabolism, especially the metabolic pathways for nucleotide synthesis, continue to emerge.”
“Identifying established malignancies in humans is usually not a difficult clinical issue as most human cancers do not become symptomatic until they are well advanced. Indeed, by the time they are detected, most will already consist of millions, if not trillions, of cells exhibiting many abnormal features.” “The complexity of these constantly evolving systems is apparent, and elucidating all of the cellular constituents— let alone their interactions with each other—is well out of reach of even the most sophisticated teams of pathologists.” “…almost all tumours accumulate a very large number of mutations over time, typically 1012 or more, both as a result of pre-existing changes in the stem cell of origin and of subsequent mutations during tumourigenesis.”
So you know:
“Each day, your immune system spots and destroys cells that could easily go on to become cancerous”. For example, typically “…cells up for destruction [may be] rogue cells from the immune system itself, called immune B cells. It’s common for some of these immune cells to undergo spontaneous changes that could lead to cancer, but the immune system quickly eliminates them.” “If the mutant immune B cells weren’t swiftly disposed of, they could develop into blood cancers called non-Hodgkin lymphomas (B-cell lymphomas)…” It is interesting that there is “ ‘surprising rarity’ of non-Hodgkin lymphomas despite how often these spontaneous changes occur in immune cells.” But, research shows that “immune surveillance by T cells enables early detection and elimination of these cancerous and pre-cancerous cells…”
Fundamentally, “Cancer is a genetic disease: it results from mutations in somatic cells. To understand it at a molecular level, we need to identify the relevant mutations and to discover how they give rise to cancerous cell behavior. Finding the mutations is easy in one respect: the mutant cells are favoured by natural selection and call attention to themselves by giving rise to tumours. The hard task then begins: how are the genes with the carcinogenic mutations to be identified among all the other genes in the cancerous cells?” “Despite these difficulties, many genes that are repeatedly altered in human cancer have been identified…although it is clear that many more remain to be discovered. We will call such genes, for want of a better term, cancer-critical genes, meaning all genes whose mutation contributes to the causation of cancer.”
So you know:
“When cancer cells grow and divide, they can move from where they started to other areas of the body. There are 3 ways cancer can spread.”“Direct extension, or invasion, means that the primary tumour grows into tissues or structures around it. For example, prostate cancer can grow into the bladder.”
“Lymphatic system spread means that cancer cells break away from the primary tumour and travel to another part of the body through the lymphatic system. The lymphatic system is a group of tissues and organs that make and store cells that fight infection and diseases.”
“Bloodstream, or hematogenous spread means that cancer cells breakaway from the primary tumour, enter the bloodstream and travel to a new place in the body.”
“The immune system usually attacks and destroys cancer cells that travel through the lymphatic system or bloodstream. But sometimes cancer cells survive and settle in another area of the body, where they form a new tumour. To survive and grow in the new location, the tumour must form its own blood supply (called angiogenesis).”
“Conversion of a normal body cell into a malignant one is now known to require multiple mutations. Three different types of experimental approaches all converged on this important conclusion: epidemiology of human cancers, analyses of DNA in cells at several stages in the development of cancers in humans and mice, and overexpression of oncogenes in cultured cells and transgenic animals.” The transformation of a normal tissue into a malignant tumour (carcinogenesis) is a multistep process, characterized by the gain or loss of gene expression and epigenetic (non-genetic) changes. Such changes lead to the acquiring of the biological characteristics that result in tumour cells progressing from a benign through various malignant phenotypes. According to Montréal researcher Dr Richard Momparler, “Epigenetics is described as a heritable change in gene expression without an alteration in the DNA sequence. Research in the past 10 years indicates that epigenetic changes play an important role in tumourigenesis. The major epigenetic changes that take place during the development of cancer are the aberrant DNA methylation of genes that suppress tumourigenesis and histone modifications of chromatin.”
So you know:
“Prostate cancer remains a leading cause of male cancer death worldwide. The mainstay of therapy for patients with metastatic spread, including castration resistant disease, is hormonal therapy targeting the Androgen Receptor.”
Still, “A growing number of researchers believe that cancer growth, metastasis, and invasion depend not only on the tumour cells themselves, but also on the growth of tumour microenvironment, which supports cancer behavior.” “Tumour masses have been compared to rogue organ systems that are comprised of collections of cells that compete with each other and with normal tissue in a Darwinian struggle for survival.” “It has long been appreciated that established tumours are complex masses that contain not only neoplastic cells but also non-transformed cellular elements such as stromal cells, the neo-vasculature, and a gamut of immune cells.”
“Conversely to normal cells, where deregulated oxidative stress drives the activation of death pathways, malignant cells exploit oxidative milieu for its advantage. Cancer cells are located in a very complex microenvironment together with stromal components that participate to enhance oxidative stress to promote tumour progression. Indeed, convincing experimental and clinical evidence underline the key role of oxidative stress in several tumour aspects thus affecting several characteristics of cancer cells. Oxidants influence the DNA mutational potential, intracellular signaling pathways controlling cell proliferation and survival and cell motility and invasiveness as well as control the reactivity of stromal components that is fundamental for cancer development and dissemination, inflammation, tissue repair, and de novo angiogenesis.”
“Metastasis is a key step in cancer progression, and was traditionally attributed to the accumulation of genetic and epigenetic changes within individual cancer cells. These changes promoted invasiveness, immune evasion and survival at distant sites. However, recent studies reveal that metastasis is not achieved by the cancer cell in isolation, but requires intervention from the immune system. The myeloid cell population in particular is now implicated in many aspects of metastasis.”
For a more developed definition of Carcinogenesis and others; see the Rolling Reference for this chapter
So you know:
“One of the biggest sources of confusion for patients comes when their cancer has spread from one location to another. For example, it is possible for breast cancer to spread to the bone. People will assume they now have bone cancer, but this is not the case. They actually have metastatic breast cancer.” “Cancer is treated by cell type. Breast cancer cells respond in certain ways to certain treatments. If cancer has spread to the bone, you will still receive treatment for breast cancer.”
“Solid cancers are heterocellular systems containing both tumour cells and stromal cells. Coercion of stromal cells by tumour cell oncogenes profoundly impacts cancer biology and aberrant tumour-stroma signaling regulates many hallmarks of cancer.” “Solid tumours have a distinct structure that mimics that of normal tissues and comprises two distinct but interdependent compartments: the parenchyma (neoplastic cells) and the stroma that the neoplastic cells induce and in which they are dispersed. In many tumours, including those of epithelial cell origin, a basal lamina separates clumps of tumour cells from stroma. However, the basal lamina is often incomplete, especially at points of tumour invasion.”
So you know:
“It’s well established that mutations in cancer cells can drive phenotypic changes within cancer cells. For example, a point mutation in a kinase gene can result in hyperactive signaling — causing a cancer cell to grow too fast. When a cell is affected by its own mutation, it’s known as a cell-autonomous event.” “When a mutation in one cell regulates the behaviour of a neighbouring cell, this is called a non-cell-autonomous event. Despite its huge role in cancer, non-cell-autonomous signaling is poorly understood. Most examples are anecdotal to a single pathway or molecule. We actually know very little about how mutations signal beyond cancer cells. It’s often presumed to occur, but we have no idea how much, and what the consequences are.”
“Tumours are strongly influenced by the surrounding normal tissue, which forms a specialized niche termed the tumour microenvironment (TME). The TME is modeled by cancer cells for their own benefit through a complex array of interactions. The identification of new forms of communication within the TME, which are dependent on the tumour’s metabolic activity, has expanded our understanding of this heterocellular regulation and has revealed potential therapeutic targets.” In fact, recent “Investigations into the role of the TME and the mechanisms of stromal cell recruitment have also provided insights into a distinct and intriguing aspect of tumour biology: cancer progression may also be directed by the body’s systemic responses to malignancy and by the involvement of organ systems located at sites distant from the site of primary tumour growth. In some cases, the systemic interactions between tumour and host mimic and co-opt normal physiological processes, such as inflammation and wound healing. Indeed, the histopathological appearance of the TME of most carcinomas closely resembles that of inflamed and wounded tissues, a similarity that was noted a quarter of a century ago when tumours were described as ‘wounds that do not heal’.” “These similarities explain why studies of other pathological processes, such as wound healing, inflammation and organ fibrosis have shed light on carcinoma pathogenesis. Importantly, however, the transient activation of stromal cells observed during wound healing contrasts with the behaviours of these cells in tumours, where stromal cell recruitment and activity persist throughout the course of tumour development.”
So you know:
“Several important signaling pathways have been identified as frequently genetically altered in cancer, including the RTK/RAS/MAP-Kinase pathway, PI3K/Akt signaling, and others. Members of these pathways and their interactions have been captured in a number of pathway databases, such as Pathway Commons (Cerami et al., 2011), which aggregates a number of ~20 databases, including REACTOME (Joshi-Tope et al., 2005) and KEGG (Kanehisa and Goto, 2000). Genes in key pathways are not altered at equal frequencies, with certain genes recurrently altered and well-known in cancer, while others are only rarely or never altered.”
This being said; however, “Carcinogenesis [must] be considered as a complex micro-evolutionary process, which requires the accumulation of a range of (somatic) genetic mutations. Under selection pressure and through these mutations, cells acquire new characteristics, which provide them with an advantage in growth behaviour and other cellular properties, such as enhanced survival and invasiveness. This process is in most cases drawn out over many years and requires a series of individual steps.” For example, “Most genetic changes that are hallmarks of epithelial cancer are already present in premalignant lesions that rarely progress to frank cancer.” as shown by “…ultra-deep sequencing of 74 cancer genes in small biopsies of normal aged and sun-exposed human skin [revealing] a high mutation burden in most key drivers of cutaneous squamous cell carcinoma.”
“Cancer cells do not look or act like the normal cells from which they originate. It is reasonable, then, to ask ‘Why do cancer cells behave so badly?’ ”
As “It turns out the answers lie in the genes of the affected cells. In cancer cells, changes to key genes cause the cells to act abnormally. The changes are often the result of changes to the DNA (mutations) in the cells. Because there are many different things that are capable of causing mutation, there are an equally large number of causes of cancer.”
So you know:
“Cell proliferation, death, and differentiation are fundamental biological processes. Coordination of these processes is critical for a wide range of physiological and pathological conditions (Pellettieri and Sanchez Alvarado 2007; Galliot and Ghila 2010). During development, an increase in cell number is required to boost organ and body size; meanwhile, proper differentiation of multiple cell types will assure the appropriate function of developed organs. In adulthood, most tissues undergo continuous cell turnover to maintain functionality. Aged or damaged cells are programmed to cell death, whereas adult stem cells may divide and differentiate to replace those dysfunctional cells. Under pathological conditions, such as wound healing and organ regeneration, cell division and differentiation of tissue-specific progenitor cells will be up-regulated to compensate for the lost cells. On the other hand, uncontrolled cell proliferation and decreased cell death lead to hyperplasia or tumourigenesis.”
“Several evidences demonstrate that embryogenesis and tumourigenesis have common characteristics, where both processes depend on coordinated mechanisms of proliferation, differentiation and migration. Vital signaling pathways for embryonic development and organogenesis are modulated in tumourigenesis.” Therefore, “Cancer is not only the transformation of individual cells into a state of cellular proliferation, but a disruption of the forms in which the tissues regulate their processes and affect the systemic interactions with the affected organism. Currently, the fundamental treatments against cancer continue to be surgery, radiation therapy, and chemotherapy, which usually destroys the primary tumour, but whose action is very limited against metastasis. This is why it is necessary to continue investigating to find new prognostic markers and new therapeutic targets for metastasis before it occurs, as the early detection of these markers could determine which cases require treatment and avoid it in those patients without a risk of metastasis. Thus, for example, the monitoring of growth factors and cytokines in the blood which may induce the formation of the pre-metastatic niche would be fundamental. At the same time, determining the blood levels of components of the metastatic niche such as the VEGFR1 protein circulating or interfering with the formation of inflammatory components such as type CD11b+ myeloid cells is indispensable for this purpose.” Though, “Recent studies have demonstrated that not all myeloid cells within TME have suppressive/regulatory functions.”

So you know:
“Blood cells, which are categorized in either the lymphoid or the myeloid lineage, are generated from hematopoietic stem cells (HSCs). Lymphoid lineage cells include T, B, and natural killer (NK) cells, while megakaryocytes and erythrocytes (MegE) as well as granulocytes and macrophages (GM) belong to the myeloid lineage. These two lineages are separable at the progenitor level. Common lymphoid progenitors (CLPs) can differentiate into all types of lymphocytes without noticeable myeloid potential under physiological conditions, although some myeloid related genes might be detected in CLPs, depending on the experimental conditions. Similarly, common myeloid progenitors (CMPs) can give rise to all classes of myeloid cells with no or extensively low levels of B-cell potential. Another cell type, dendritic cells (DCs), is not clearly grouped either in lymphoid or myeloid lineage, because DC can…arise from either CLPs or CMPs.”“Dendritic cells are highly adapted to their role of presenting antigen and directing immune responses. Developmental studies indicate that DCs originate independently from monocytes and tissue macrophages. Emerging evidence also suggests that distinct subsets of DCs have intrinsic differences that lead to functional specialisation in the generation of immunity.”
“The TME is well represented by cells of the innate and adaptive immune systems which contribute to tumour development and immune evasion.” In fact, “…immune infiltrates vary considerably from tumour to tumour and that they evolve over time.” “The TME results from an interaction between tumourigenesis and an individual’s responses to tumourigenesis. The TME is formed by interactions between tumour cells, immune cells, and cancer associated stromal cells however, there may be other factors that have not yet been identified. In addition, the TME is comprised of non-cellular components such as cytokines, chemokines and other factors released or created by the extracellular matrix.” “…many cytokines contribute to carcinogenesis, their pro- or antitumoural roles depend on the balance of these different inflammatory mediators and the stage of tumour development. For this reason, studying the role of these mediators in different tumours or stages of development is essential for designing new personalized treatments using these potential therapeutic targets.” “Until recently, inflammatory chemokines were viewed mainly as indispensable ‘gate keepers’ of immunity and inflammation. However, updated research indicates that cancer cells subvert the normal chemokine system and these molecules and their receptors become important constituents of the tumour microenvironment with very different ways to exert tumour-promoting roles.”
So you know:
“MAPK pathways relay, amplify and integrate signals from a diverse range of stimuli and elicit an appropriate physiological response including cellular proliferation, differentiation, development, inflammatory responses and apoptosis in mammalian cells.” “The regulation of cell proliferation in multicellular organism is a complex process, which is primarily regulated by external growth factors provided by surrounding cells. The MAPK pathways involving a series of protein kinase cascades play a critical role in regulation of cell proliferation”
“Mutations in the genomes of tumour cells generate neoantigens that can be recognized by T cells. Despite this immune recognition, growing tumour cells evade immune-mediated destruction to establish primary lesions and to colonize distant and diverse metastatic environments.” “…cancer cells not only make themselves ‘invisible’ to the immune system, but also favour the formation of an immunosuppressive microenvironment unable to eliminate cancer cells. As a result, the reduced secretion of molecules acting as tumour-promoting factors and the normalization of the tumour microenvironment are main goals to develop appropriate antitumour strategies.” “Tumours can be targeted with checkpoint modulators or the transfer of T cells against mutated antigens, potentially mediating the complete and durable destruction of tumours. However, a critical challenge for novel cancer therapies is elucidating mechanisms of immune escape from these therapeutic interventions.”
So…you want more?
“Oncogene-driven expression of cytokines critical for the recruitment and phenotype of immune cells, particularly cells of the myeloid lineage, has been reported. In human melanoma, BRAF V600E, a mutated and highly oncogenic form of the MAPK family member BRAF, and STAT3, a potent transcriptional regulator often linked to oncogenic signaling, have been shown to drive expression of IL-6, IL-10 [Interleukins 6 & 10] and VEGF, cytokines that promote a tolerogenic monocyte-derived DC [Dendritic Cell] phenotype in vitro, a process that would theoretically affect antitumour T cell function in vivo.” And, “Multiple reports have demonstrated that KRASG12D driven PDAC [Pancreatic Ductal Adenocarcinoma] secretes high levels of the growth factor GM-CSF [Granulocytemacrophage Colony-Stimulating Factor], which is associated with an increase in tumour-associated Gr-1+CD11b+ myeloid cells of reported immunosuppressive function.” “Recent data in a BRAF V600E and Pten-deficient mouse model of melanoma suggest that constitutive tumour-intrinsic WNT/β-catenin signaling is associated with poor immune infiltration and ineffective antitumour T cells, largely because of a decrease in the recruitment and frequency of CD103+ DCs. Transcriptional analysis of tumour cells and in vitro DC migration assays have revealed that constitutive WNT/β-catenin signaling leads to decreased production of CCL4, a potent chemoattractant for a variety of myeloid cells including CD103+ DCs, thus potentially explaining the decreased recruitment of CD103+ DCs and the corresponding poor infiltration of CD8+ T cells into the tumour microenvironment. Although the direct oncogenic determinant of expression is unclear, several studies in mice have reported that tumour-secreted CCL2 causes the recruitment of CCR2+ classical monocytes to the tumour, where they differentiate into TAMs [Tumour-Associated Macrophages], a protumoural myeloid population.” “Dendritic cells (DCs) play a central role in the regulation of the balance between CD8+ T cell immunity vs. tolerance to tumour antigens. Cross-priming, a process which DCs activate CD8+ T cells by cross-presenting exogenous antigens, plays a critical role in generating anti-tumour CD8 T+ cell immunity. However, there are compelling evidences now that the tumour microenvironment (TME)-mediated suppression and modulation of tumour-infiltrated DCs (TIDCs) impair their function in initiating potent anti-tumour immunity and even promote tumour progression.”“Resident memory T cells (TRM) inhabit peripheral tissues and are critical for protection against localized infections. Recently, it has become evident that CD103+ TRM are not only important in combating secondary infections, but also for the elimination of tumour cells. In several solid cancers, intratumoural CD103+ CD8+ tumour infiltrating lymphocytes (TILs), with TRM properties, are a positive prognostic marker.” “The anti-tumour functions of CD8+T cells in the TME are dependent on antigen presentation; successful T cell priming, trafficking, differentiation and function. Naïve CD8+ T cells migrate from the bone marrow and secondary lymphoid organs; differentiate from naïve CD8+ T cells to effector CD8+T cells and further differentiate into memory and cytotoxic cells. Cytotoxic CD8+ T cells enter the tumour site to perform their function and memory CD8+ T cells may either enter the tumour site as TRM cells or recirculate in the blood to perform their various functions as TCM [Central Memory T] cells. In theory, once cytotoxic CD8+ T cells enter the tumour site they should be able to destroy tumour cells however, the cells and factors of the TME provide an immunosuppressive environment preventing the function of CD8+ T cells. Various forms of cancer immunotherapy have proven to be effective in re-establishing and promoting CD8+T cell anti-tumour immunity however, many of these therapies have a short-lived and or limited effectivity due to the immunosuppressive effects of the TME. Combination therapies focusing on upregulating and improving effector CD8+ T cell function as well as those impeding immunosuppressive effects of the TME are of increasing vital significance in cancer immunotherapy. In addition, preventing and reversing T cell exhaustion and enhancing the stem-cell like properties of CD8+T cells are promising mechanisms to improve CD8+T cell proliferation and survival within the TME, enhance anti-tumour function, as well as valuably improve adjunct modifications to adoptive T cell therapies in future.”
Resident memory T cells “TRM are present in many human cancers (NSCLC, ovarian cancer, bladder cancer, endometrial cancer, melanoma, etc.). Overall, they are associated with a good clinical outcome. Interestingly, the impact of TRM on survival was independent of the infiltration of CD8+ T cells.” A “…novel population of memory CD8+ T cells…has been identified based on their phenotype (CD103, CD69) and on their local tissue residency without recirculating in the blood. These cells have been implicated in protective immune response against pathogens in both animal models and humans.”
“Cytokines and colony-stimulating factors include a variety of mediators that are normally present in the human body. Immunomodulatory cytokines include interferons (IFNs), interleukins (IL)-2, IL-12, IL-15 and IL-18, colony-stimulating factors and tumour necrosis factor (TNF) alpha.”
So you know:
“There are two types of remission:”
- “Partial remission means the cancer is still there, but your tumour has gotten smaller — or in cancers like leukemia, you have less cancer throughout your body. Some doctors tell patients to think of their cancer as ‘chronic’, like heart disease. It’s something you will need to continue to check. If you’re in partial remission, it may mean you can take a break from treatment as long as the cancer doesn’t begin to grow again.”
- “Complete remission means that tests, physical exams, and scans show that all signs of your cancer are gone. Some doctors also refer to complete remission as ‘no evidence of disease (NED)’. That doesn’t mean you are cured.”
“Some cancer cells can remain unnoticed in the body for years after treatment. If a cancer returns after it has been in remission, it’s called a ‘recurrence.’”
“The interferons (IFN) are glycoproteins that are produced by cells in response to viral infection. IFNs are so called because they ‘interfere’ with subsequent viral challenges to noninfected cells. There are three types of IFN:”
- “Alpha: Produced by macrophages and lymphocytes.”
- “Beta: Produced by fibroblasts and epithelial cells.”
- “Gamma: Produced by CD4+, CD8+, NK, and LAK cells.”
“Interferons bind to cell membrane receptors and activate molecular and cellular events. Different mechanisms of action contribute to the antitumour effect of IFNs:”
- “Antiproliferative effects.”
- “Promotion of differentiation.”
- “Immunomodulation, e.g., potentiation of tumour cytotoxicity of TNF; increased ratio of CD4+ to CD8+ cells.”
- “Alteration in tumour cell surface antigen expression, e.g., induction of MHC expression on tumour cell surfaces.”
- “Inhibition of oncogene activation.”
- “Inhibition of angiogenesis.”
- “Enhancement of macrophage activity.”
So you know:
LAK cells are “Killer cell lymphocytes activated in the presence of interleukin-2 (IL-2). Lymphokine-activated killer cells (LAKs) are cytotoxic effector cells with an exceptionally wide target cell spectrum including normal and malignant cells of different origins. LAK cells exhibit a profound heterogeneity with regard to phenotype surface marker expression; it remains to be determined if they represent a unique cell lineage.”
“Interleukin (IL)-2 is a glycoprotein produced by T lymphocytes after they have been in contact with APCs [Antigen Presenting Cells]. IL-2 is used in vitro for generation of LAK cells (specifically, a mixture of NK cells and CD4/CD8 T cells). Infusion of LAK cells together with concurrent administration of IL-2 has demonstrated beneficial effects in preclinical and clinical studies. Aldesleukin is an example of an IL-2 available in Canada. It is indicated for the treatment of patients with melanoma and renal cancer.”
So you know:
“The vast majority of cancer-related deaths are due to metastatic tumour growth that impairs the function of vital organs. Metastatic lesions invariably originate from disseminated tumour cells, which often undergo a period of dormancy. Cancer recurrence after therapy and long periods of remission is frequent. For example, 20–45% of patients with breast or prostate cancer will relapse years or decades later. In fact, most cancer types are associated with disseminated disease that after treatment might persist as minimal residual disease. However, the lack of mechanistic insight into this stage has been a major shortcoming in our understanding of the full complexities of metastatic growth. Functional characterization of disseminated dormant tumour cells is important because these cells most probably contain the information about the future progression of the disease (that is, metastasis development). To fully understand dormancy, cells must be characterized during the dormant state.” “Cancer dormancy can be separated into mechanisms that antagonize the expansion of a dividing tumour cell population (tumour mass dormancy) and mechanisms that result in tumour cell growth arrest (tumour cell dormancy, or cellular dormancy). In the former, tumour cells usually divide but the lesion does not expand beyond a certain size because of either limitations in blood supply or an active immune system. Cellular dormancy can occur when tumour cells enter a state of quiescence.” “These general mechanisms might explain the dormancy of residual cells that, following treatment, develop loco-regional or distant organ recurrences within different time frames.”“Although your 40 trillion cells all have the same genome, your body actually contains over 200 different types of cell. Each cell type differentially expresses disparate proteins to enable unique emergent phenotypes. Your cells are differentiated. Consider something as simple as your skin. It’s not just made of ‘skin cells’. Skin is not homocellular. The first 0.5 mm alone contains keratinocytes, melanocytes, dendritic cells, Merkel cells, and lymphocytes. Tissues are heterocellular. Heterocellularity allows tissues to achieve multiple phenotypes from a common genome. In your skin, keratinocytes form a barrier against the outside world, while your lymphocytes fight off infections. Same genotype, differentiated phenotypes.” “Coordinating heterocellularity requires constant communication between different cell types. This is called ‘heterocellular signaling’ and it’s essential for all metazoan life. Heterocellular Signaling is also frequently dysregulated in disease. Cancer, neurodegenerative disorders, and infectious disease all involve disrupted heterocellular Signaling. If we want to combat these conditions, we need to understand how different cells communicate. Despite the importance of heterocellular Signaling, it’s experimentally awkward to study.” “Over the past few years several nascent techniques have emerged that now enable systems biology analysis of heterocellular Signaling. These techniques have facilitated pioneering studies of heterocellular Signaling and described unique intercellular communication events.”
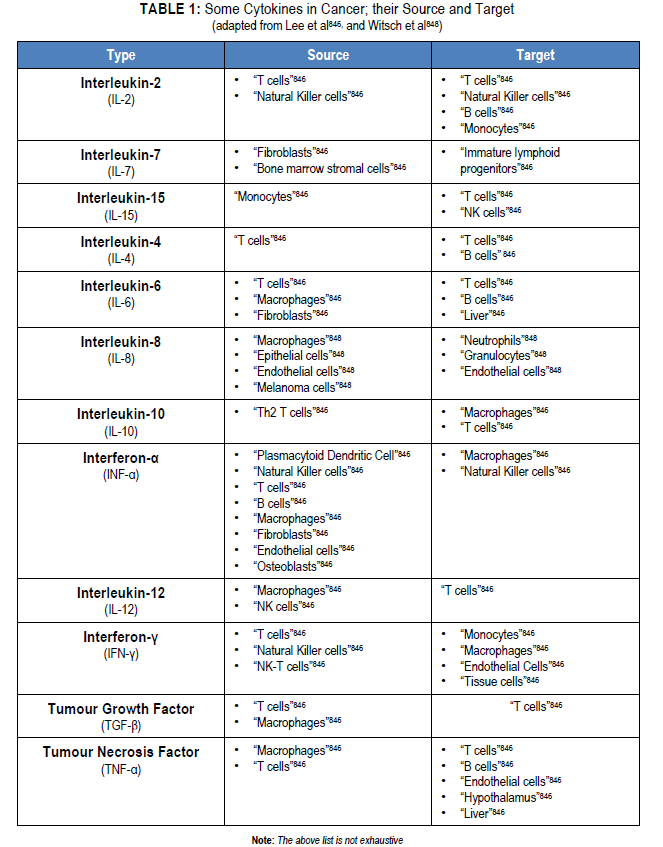
“Cytokines play an important role in host defense against microorganisms. They orchestrate innate immunity by inducing protective local inflammation and systemic acute phase responses. Cytokines are important in initiating, amplifying, directing, mediating, and regulating adaptive immunity. Unfortunately, they may also direct tissue damage if excessive responses occur or if they are involved in directing and mediating autoimmunity. Under these circumstances, cytokines are potential therapeutic targets.”
So you know:
“The size of an organ or organism depends on the cell size and the number of cells. The number of cells present in an organ correlates with the cell’s capacity for cellular division/proliferation and cell death. There are several classes of extracellular signals and intracellular programs that contribute to the control of cell size, cell proliferation and cell death. These include mitogens, growth factors, survival factors and apoptotic signals.”“The tumour is not an autonomous entity composed entirely of genetically altered cells; it also contains numerous genetically unaltered cell types. Collectively, all these various cell types form a complex ecosystem. Cancer-associated fibroblasts (CAFs), immune and endothelial cells, and other elements with their products, including the extracellular matrix, reciprocally interact with malignant cells within the tissue microenvironment and in such a way influence the biological properties of tumours. As space and nutrition are limited, normal and cancer cells, including different cancer clones, complete with each other, resulting in tumour evolution. All these factors include, but are not limited to local aggressiveness and metastasis formation in a cancer type-dependent manner. Therefore, targeting the ecosystem/niche rather than the cancer clone alone may have an important clinical implication based on tumour biology. Specific molecules targeting selected steps in angiogenesis, extracellular matrix formation, CAF biology, several signaling pathways, growth factors and the immune system were experimentally considered to be effective in the modulation the tumour microenvironment (TME), thus have been selected for several clinical trials.”
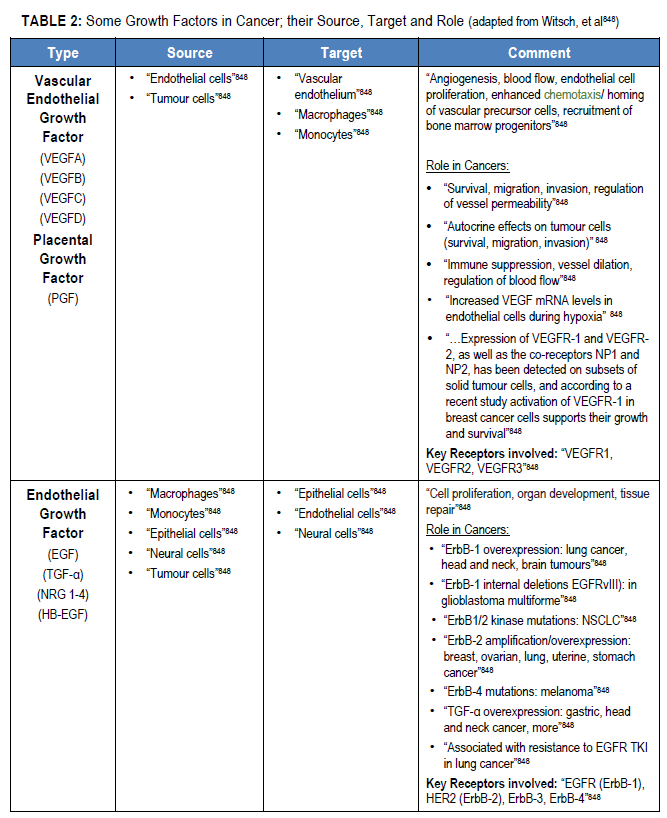
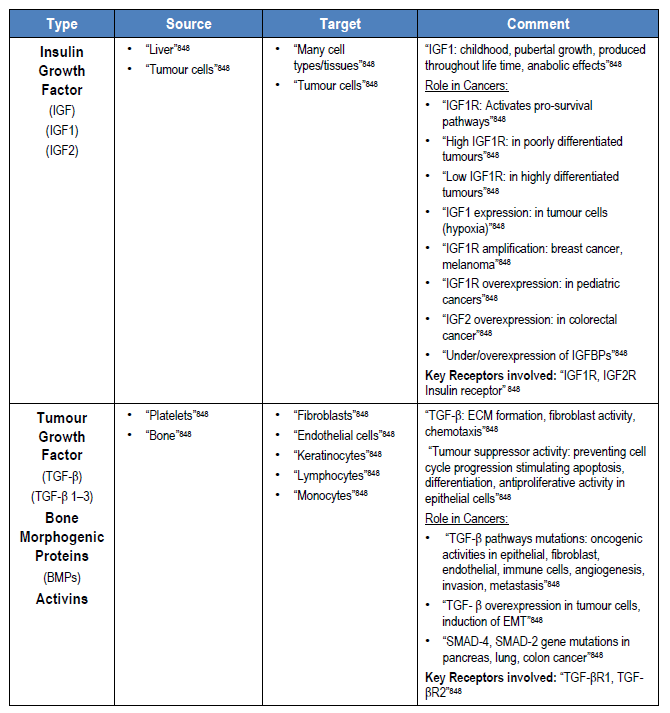
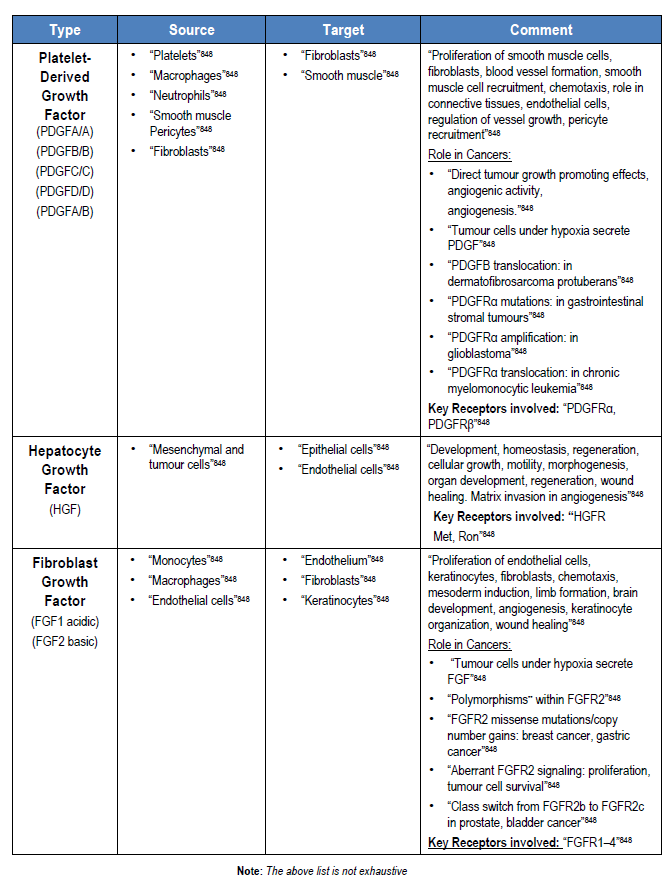
“Under physiological conditions, cells receive fate-determining signals from their tissue surroundings, primarily in the form of polypeptide growth factors. Integration of these extracellular signals underlies tissue homeostasis. Although departure from homeostasis and tumour initiation are instigated by oncogenic mutations rather than by growth factors, the latter are the major regulators of all subsequent steps of tumour progression, namely clonal expansion, invasion across tissue barriers, angiogenesis, and colonization of distant niches”
**Polymorphism: “A common change in the genetic code in DNA. Polymorphisms can have a harmful effect, a good effect, or no effect. Some polymorphisms have been shown to increase the risk of certain types of cancer.”
So you know:
“Growth factors promote an increase in cell growth. Some growth factors act as both mitogens and growth factors at the same time. Extracellular growth factors bind to cell membrane receptors, and activate tyrosine kinases and intracellular signaling pathways, which leads to increasing the rate of synthesis of proteins and/or decreasing the rate of protein degradation. Growth factors may also stimulate the production of the gene regulatory protein Myc that plays an important part in signaling by mitogens. For example, the epidermal growth factor family of tyrosine kinases plays a key role during embryonic development and in the maintenance of adult tissues.”
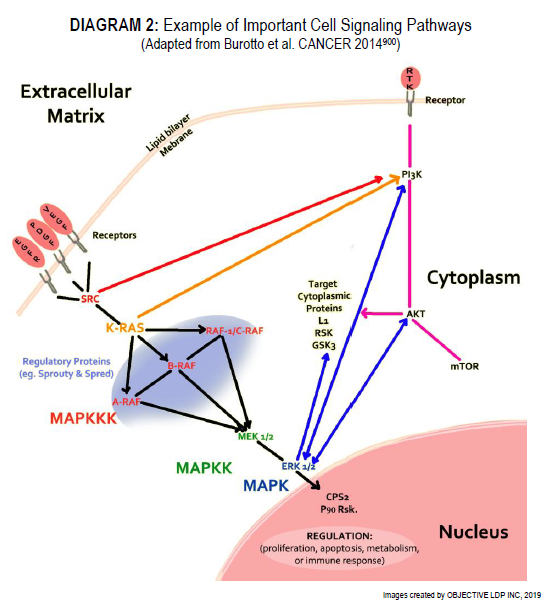
So…you want more?
Kindly refer to DIAGRAM 2:
“Growth factors and mitogens use the Ras/Raf/MEK/ERK signaling cascade to transmit signals from their receptors to regulate gene expression and prevent apoptosis. Some components of these pathways are mutated or aberrantly expressed in human cancer (e.g., Ras, B-Raf).” “Signaling pathways are somatically altered in cancer at varying frequencies and in varying combinations across different organs and tissues, indicative of complex interplay and pathway cross-talk. Understanding the extent, detailed mechanisms, and co-occurrence of the oncogenic alterations in these pathways is critical for the development of new therapeutic approaches that can improve patient care.”
“Excessive cell proliferation is a feature of most cancers. Limited availability of growth factors or nutrients, contact inhibition, and other feedback mechanisms ensure that the pathways that regulate proliferation are normally tightly controlled.” “…however, mutations in proto-oncogenes and tumour suppressors or inappropriate synthesis of ligands/receptors can hyperactivate these pathways, leading to activation of the cell cycle machinery.” “Recent evidence indicates that the MAPK/ERK signaling node can function as a tumour suppressor as well as the more common pro-oncogenic signal. The effect that predominates depends on the intensity of the signal and the context or tissue in which the signal is aberrantly activated. Genomic profiling of tumours has revealed common mutations in MAPK/ERK pathway components, such as BRAF.” “The Ras-ERK and PI3K-Akt pathways are important regulators of normal cell proliferation and thus their constitutive hyperactivation can lead to excessive proliferation.”
“The Ras-mitogen-activated protein kinase (MAPK) pathway is an important regulator of diverse cellular processes.” “After membrane receptor activation, adaptor proteins recruit RAS proteins to activate steps concluding with ERK activation. Successive steps of phosphorylation amplify the signal, Raf→MEK→ERK, until ERK activates its cytoplasmic and/or nuclear targets. Regulatory phosphatases, Sprouty and Spred, modulate the intensity of the signal. The PI3K-AKT pathway interacts with the MAPK/ERK node under normal conditions and in the cancer cell. Target cytoplasmic proteins include RSK, ribosomal S6 kinases; GSK3, glycogen synthase kinase 3; L1, adhesion molecule L1. Additional proteins in nucleus include CPS2, p90Rsk”.
“The Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt pathways interact with each other to regulate growth and in some cases tumourigenesis. For example, in some cells, PTEN mutation may contribute to suppression of the Raf/ MEK/ERK cascade due to the ability of activated Akt to phosphorylate and inactivate different Rafs. Although both of these pathways are commonly thought to have anti-apoptotic and drug resistance effects on cells, they display different cell lineage specific effects. For example, Raf/MEK/ERK is usually associated with proliferation and drug resistance of hematopoietic cells, while activation of the Raf/MEK/ERK cascade is suppressed in some prostate cancer cell lines which have mutations at PTEN and express high levels of activated Akt. Furthermore, the Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt pathways also interact with the p53 pathway.”
“Mesenchymal stem cells (MSCs) [also] represent an interesting population due to their capacity to release a variety of cytokines, chemokines, and growth factors, and due to their motile nature and homing ability. MSCs can be isolated from different sources, like adipose tissue or bone marrow, and have the capacity to differentiate, both in vivo and in vitro, into adipocytes, chondrocytes, and osteoblasts, making them even more interesting in the regenerative medicine field. Tumour associated stroma has been recognized as a key element in tumour progression, necessary for the biological success of the tumour, and MSCs represent a functionally fundamental part of this associated stroma.”
So you know:
“Ligand binding to the receptor allows for signal transduction through the cell. The chain of events that conveys the signal through the cell is called a signaling pathway or cascade. Signaling pathways are often very complex because of the interplay between different proteins. A major component of cell signaling cascades is the phosphorylation of molecules by enzymes known as kinases. Phosphorylation adds a phosphate group to serine, threonine, and tyrosine residues in a protein, changing their shapes, and activating or inactivating the protein.”
“Exosomes represent one of the dominant signaling pathways within the tumour microenvironment. Their biology raises high interest, with implications in different biological processes involved in cancer progression, such as the formation of the pre-metastatic niche. This is critical during the metastatic cascade, given that it is the formation of a permissive context that would allow metastatic tumour cells survival within the new environment.” “The function of exosomes depends on the type of cells from which they are derived. Studies have shown that tumour-derived exosomes are involved in the exchange of genetic information between tumour cells and basal cells, resulting in the production of a large number of new blood vessels, which promotes tumour growth and invasion.”
So…you want more?
“Chromosome instability [CIN] describes an increased rate of chromosome mis-segregation in mitosis resulting in an incorrect chromosome number and/or abnormal chromosome structure (Rao et al. 2009). Although CIN has been long recognized as a hallmark of a majority of tumours, it remains inconclusive if CIN is an early step or a final demonstration of cancer progression. Equal segregation of chromosomes during mitosis is pivotal for the maintenance of genomic stability. Failure of accurate chromosome segregation inevitably leads to cell death or malignant transformation. Accurate chromosome segregation during cell division is monitored and safeguarded by several closely linked yet distinctly different molecular machineries.”
“A pre-metastatic niche is a microenvironment prepared for the colonization of circulating tumour cells in specific organs.” “The key components of the pre-metastatic niche include tumour-derived secreted factors (TDSFs), extracellular vesicles (EVs), bone marrow-derived cells (BMDCs), suppressive immune cells and host stromal cells.” “It is known that cancer cells communicate with fibroblasts via EVs, and this leads to the progression of metastasis. The fibroblasts in tumours are involved in epithelial-mesenchymal transition (EMT) and chemotherapy resistance by contacting…cancer cells and [with] each other.” “It is now apparent that MDSC play an important role in tumour progression by regulating different aspects of the immune response in cancer. The effect of MDSC depends on their localization. MDSC generated in the BM [Bone Marrow] migrate to peripheral lymphoid organs and tumours.”
So…you want more?
“The endocytic network comprises a vast and intricate system of membrane-delimited cell entry and cargo sorting routes running between biochemically and functionally distinct intracellular compartments. The endocytic network caters to the organization and redistribution of diverse subcellular components, and mediates appropriate shuttling and processing of materials acquired from neighbouring cells or the extracellular milieu. Such trafficking logistics, despite their importance, represent only one facet of endocytic function. The endocytic network also plays a key role in organizing, mediating, and regulating cellular signal transduction events. Conversely, cellular signaling processes tightly control the endocytic pathway at different steps.”
“Genetic, cellular biology, and molecular studies, as well as those of the internal and external environmental contexts, indicate that tumour growth is not only determined by its cells, but also by the tumour microenvironment and the entire context in which the organism functions. In this way, the progression of cancer is the result of a very complex relationship between the different malignant and non-malignant cell types, components of the stroma, and the entire body of the organism.” It is more than clear that “Primary and metastatic tumours are complex ecosystems composed of neoplastic cells, extracellular matrix (ECM), and ‘accessory’ nonneoplastic cells, which include resident mesenchymal support cells, endothelial cells, and infiltrated inflammatory immune cells. Crosstalk between cancer cells and accessory cells fuels and shapes tumour development. During tumour formation, the tissue architecture evolves into a highly specialized microenvironment characterized by a corrupted ECM and chronic inflammation.” In fact now, “It is well known that the crosstalk between cancer cells and stromal components in many tumour types can enhance tumour growth and metastasis.”
So you know:
“Metastasis causes more than 90% of cancer-related deaths. For carcinomas, the most common type of tumours, metastasis begins when some epithelial cells from the primary tumour lose their apico-basal polarity and cell-cell adhesion and acquire migratory and invasive characteristics, through a process known as Epithelial–to-Mesenchymal Transition (EMT).”“Correct establishment and maintenance of cell polarity is required for the development and homeostasis of all metazoans. Cell-polarity mechanisms are responsible not only for the diversification of cell shapes but also for regulation of the asymmetric cell divisions of stem cells that are crucial for their correct self-renewal and differentiation. Disruption of cell polarity is a hallmark of cancer.”
“Tissue stiffness is tightly controlled under normal conditions, but changes with disease. In cancer, tumours often tend to be stiffer than the surrounding uninvolved tissue…” “Within the past decade, and particularly in the last few years, there is increasing evidence that the stiffness of the extracellular matrix modulates cancer and stromal cell mechanics and function, influencing such disease hallmarks as angiogenesis, migration, and metastasis.”
“Therapeutic decisions for cancer patients are primarily based on clinical and pathological parameters. In particular, tumour size, histological grade, histotype and immunohistochemical results of prognostic factors play major roles in planning therapeutic strategies (e.g., targeted therapy or chemotherapy). Although this has been a successful approach, many patients relapse and/or eventually develop resistance. Despite the fact that vast technological improvements have increased our understanding of human cancers as heterogeneous diseases, current clinicopathological, immunohistochemical and molecular parameters/markers leave significant numbers of patients at risk for over- or under-treatment.”
“Advances in early detection and the development of new treatment options such as targeted combination therapies and immunotherapies have increased survival rates for lung cancer patients over the last decade. However, many of these advances have associated side effects that can present during the course of the disease and even after active treatment. It is important to address the longitudinal effects of treatment and recognize the need for supportive care interventions for patients. Many treatment options available in standard care or clinical trials are accompanied by known side effects. Advances in supportive care have changed the cancer experience for many patients. Supportive care is a valuable part of the success of treatment and helps to provide positive outcomes. As a result, practitioners are better prepared to address and prevent cancer-related symptoms.”
“Supportive care is a term that refers to treatment that aims to decrease or eliminate symptoms associated with cancer. The goal is to maximize comfort, minimize suffering, and ensure the highest quality of life. Supportive care focuses on treating cancer-related symptoms, preventing and managing treatment-related side effects, recognizing and supporting psychosocial distress, and helping to develop strategies for improving quality of life. Comprehensive supportive care may address symptoms that occur at diagnosis and during or after treatment.” “Supportive care is important throughout the continuum of cancer care. Supportive care needs may change during the course of the disease and assessment, including diagnosis, treatment, survivorship, and end of life. People living with cancer may experience varied symptoms during the course of the disease, such as more psychological concerns and symptoms at the time of diagnosis than at later stages of treatment. However, physical symptoms may become an immediate concern during treatment. As the disease and physical symptoms progress, patients may experience difficulties in coping with the situation. Patients with advanced cancer or disease progression must address a change or deterioration of physical health, resulting in psychological and social concerns. The management of these symptoms and psychological distress is important to optimize quality of life.”
“Being diagnosed with…cancer is a life-changing event that can have a profound effect on the physical, emotional, and psychosocial aspects of one’s well-being. [Sometimes], “there are many symptoms and side effects associated with…diagnosis and treatment. These symptoms can interfere with the ability to function and perform daily activities, decreasing the patient’s quality of life, especially if symptoms are ignored and go untreated.” For example, “Lung cancer patients have more unmet supportive care needs than patients with other cancers. Lung cancer is often associated with a heavy disease burden, and patients can derive benefit from supportive care interventions, thus limiting impact. Supportive care interventions can improve well-being and survival for cancer patients. Intervening early may decrease unnecessary suffering and enable patients to feel strong enough to be active participants in their cancer care. The goal of supportive care is to provide patients with the best quality of life throughout the cancer experience, enabling them to perform daily activities and engage in activities that bring them joy and happiness.” “A cancer diagnosis generates feelings of sadness, anger, anxiety, and fear. Patients and families struggle with quickly having to define, put into context, comprehend, and make important decisions. The initial adaptation to a diagnosis can be influenced by pre-existing psychological factors. Patients who have a past history of depressive disorders (diagnosed or undiagnosed) should be carefully monitored throughout the cancer course because the events associated with the diagnosis serve as triggers for depression. A history of depressive disorders can be worsened or aggravated by the cancer course. People deal with their diagnosis in the context of their social environment, and the social support system can positively or negatively influence how a patient copes with the illness.”
“Payer management of oncology is in flux, as evolving…coverage and payment initiatives continue to provide private [and public] health plans with potential new direction in managing the cost of cancer treatments. These changes are taking place against a background of new oncologic product introductions, rapid expansions of indications for existing therapies, and controversies over reimbursement methodologies. Management of and payment for oncologic agents is further complicated by the lack of data examining the impact of reimbursement policies on oncology outcomes. As payers seek to keep pace with ground breaking changes in the oncology arena, it is critical that they have a solid understanding of how oncology clinical trial endpoints can or should be used to guide decisions about the care that patients receive.”
In order to help keep one’s point of view as broad as possible when it relates to finding the most appropriate perspective on cancer, an appraisal of the following researchers’ positions might be in order:
- “Solid tumours can be considered as aberrant organs, which have undergone molecular and cellular reprogramming, promoting a proliferative and invasive niche, ideal for cancer cell propagation and homing at metastatic sites.”
- “In cancer research, both numerical (aneuploidy) and structural CIN [Chromosome Instability] have been shown to impact carcinogenesis and possibly therapeutic responses; however, although CIN has been associated with cancer therapy, contradictory findings have been reported regarding its implications for the therapeutic response.” “It has been demonstrated in several cancer types that CIN-mediated intra-tumoural variability is associated with increased disease aggressiveness, a phenomenon that arises as a consequence of tumour heterogeneity, or the presence of multiple cell clones at the genetic level, which makes the tumour more adaptable and better prepared to evolve resistance.”
- “The current cancer treatment paradigm is to inhibit biological pathways that are hyperactive in cancer cells with pharmaceutical reagents. While these approaches have proven successful in the clinic, they share two common limitations.”
- “First, the targeted proteins or pathways are likely to play important physiological roles in some normal tissues, and their inhibition thus leads to toxic side effects.”
- “Second, cancer cells have defective DNA damage/repair checkpoint(s) which make them genetically unstable. Consequently, cancer cells are genetically heterogeneous, and each cell contains numerous pre-existing mutations that are not normally selected.”
- “Systemic therapy creates an environment for the selection of cancer cells with mutated target proteins that no longer interact with the drug. Therefore, side effects and secondary mutation-related drug-resistance are two inevitable consequences of current cancer treatment approaches.”
- “Although, clinically available morphological, cytogenetic and genome-wide expression analysis are available to monitor the evolution of several cancer types at a single patient level, application of the evolutionary theory may provide new philosophical inspiration for further development of personalized cancer treatment.”
- “Malignant genetically altered cancer cells together with the TME form a functional ecosystem promoting the growth and spreading of tumours. Therefore, targeting the TME rather than the cancer cell itself may have an important clinical implication in stopping tumour evolution in terms of acquisition of resistance to the applied therapy or to the formation of distant metastases and thus be the hallmark of personalized cancer therapy.”
- “…a growing body of evidence supports the hypothesis that effective cancer therapies should include elimination of tumour cells, inhibition of tumour-associated angiogenesis, and subversion of tumour-induced immunosuppression by enhancing infiltration and activation of conventional DCs, targeting immunosuppressive cells, and reactivating tumour-inhibited effector T cells…”
- “Supportive care is important throughout the continuum of cancer care. Supportive care needs may change during the course of the disease and assessment, including diagnosis, treatment, survivorship, and end of life. Patients with advanced cancer or disease progression must address a change or deterioration of physical health, resulting in psychological and social concerns. The management of these symptoms and psychological distress is important to optimize quality of life.”
- “Ideally, when reviewing an oncology agent for coverage, a payer should augment the review of safety and efficacy data with a thorough cost/benefit analysis, weighing the costs of the agent against the benefits to payers, healthcare providers, and patients. Such an analysis, however, often is not possible because of a paucity of supporting data. Payers, therefore, should have a clear understanding of oncology clinical trial endpoints to make accurate decisions about the value of various treatments.”
Finally, should this not be an over-arching principle to be always kept in mind as one moves forward on the topic of cancer?
- “Due to the implication of metastasis in mortality due to cancer, it is…necessary to search for new ways to integrate the two focuses which dominate the current science: the reductionist vision and the systemic vision sustained by the science of complexity.”
So you know:
As previously considered, “Epithelial-mesenchymal transition [EMT] is the process by which epithelial cells lose cell-cell junctions and baso-apical polarity and acquire plasticity, mobility, invasive capacity, stem-like characteristics, and resistance to apoptosis. This cell biology program is active in embryology, wound healing, and pathologically in cancer metastasis, and along with the mechanical and cellular components of the tumour microenvironment, provides critical impetus for epithelial malignancies to acquire metastatic capability.” “Polarity of the epithelium serves as a barrier for tumour formation in epithelial tissue by maintaining tissue organization and 3D architecture. The loss of cell polarity and tissue organization is a hallmark of carcinoma. Invasion and metastasis are the major cause of cancer related morbidity and mortality (Hanahan and Weinberg 2011) and appear to be dependent on EMT. EMT is an important hallmark of carcinoma, (Royer and Lu 2011) where migrating tumour cells recapitulate developmental process of EMT by altering the epithelium structure, disrupting the basal lamina and invading the underlying tissues. Loss of integrity of tight and adherence junctions and loss of polarity are crucial features of EMT.” “…cell–cell contact is maintained and regulated by several polarity proteins. These polarity proteins are often targeted by EMT inducers, leading to their altered function, ultimately facilitating cell migration (Martin-Belmonte and Perez-Moreno 2012).”
THE SO-FAR SUMMARY
~ One ~
Though updated in 2011 to include four more characteristics; what made this original document so important to medicine was that for the first time, researchers had presented and argued the scientific facts as foundational for six common traits of cancer that included:
- “Cancer cells stimulate their own growth (self-sufficiency in growth signals).” “They resist inhibitory signals that might otherwise stop their growth (insensitivity to anti-growth signals).”
- “They resist their programmed cell death (evading apoptosis).”
- “They can multiply indefinitely (limitless replicative potential).”
- “They stimulate the growth of blood vessels to supply nutrients to tumours (sustained angiogenesis).”
- “They invade local tissue and spread to distant sites (tissue invasion and metastasis).”
Cancer is not a single disease, and it should be noted well that there are over 200 different types of human cancer. Some main-line kinds may be named anatomically – according to the organ involved (eg. breast, lung, bone, paranasal sinus etc.) or histologically – for the type of cell tissue out of which it arises (eg. carcinoma, sarcoma, etc.) or as it specifically relates to its original ‘discoverer’ (eg. Hodgkin, Kaposi, Ewing); or perhaps, another disease process all together – like AIDS-related cancers.
“Until recent years, the principal focus in cancer research has mostly been the malignant cell itself. As a consequence, today, there is a significant discrepancy between the vast knowledge about cancer biology generated in experimental settings and the translation of this knowledge into information that can be used in clinical decision making. Understanding the nature of the tumour environment today may be equally important for future cancer therapies as understanding cancer genetics per se. Cancers are not simply autonomous neoplastic cells but [are] also composed of fibroblasts, immune cells, endothelial cells, and specialized mesenchymal cells. These different cell types in the stromal environment can be recruited by malignant cells to support tumour growth and facilitate metastatic dissemination.” For example, the “ECM [Extracellular Matrix] is a major component of tumour microenvironment [TME] and plays critical roles in cancer development and progression.” requiring “extensive reorganization of extracellular matrix.” so that “Increased ECM proteins deposition and crosslink provide necessary biochemical and biophysical cues to promote cancer cell proliferation, migration, and invasion.”
“…cancer evolves as the result of an accumulation of mutations within a single cell. Cells have enzymes whose function is to repair defects in DNA. The genes that encode for these enzyme repair mechanism [DNA repair Enzymes] can also be damaged by mutations. In addition, there are several inherited defects in DNA repair that increase the likelihood of transition to malignancy. For example, xeroderma pigmentosum is an inherited defect in DNA repair that causes extreme sensitivity to ultraviolet light, making them extremely vulnerable to sunburn and skin cancers. Ultraviolet light penetrates the superficial layers of the skin, and causes damage to DNA. when the radiant energy is absorbed.”
“Cancer cells exhibit a dynamic metabolic landscape and require a sufficient supply of nucleotides and other macromolecules to grow and proliferate. To meet the metabolic requirements for cell growth, cancer cells must stimulate de novo nucleotide synthesis to obtain adequate nucleotide pools to support nucleic acid and protein synthesis along with energy preservation, signaling activity, glycosylation mechanisms, and cytoskeletal function. Both oncogenes and tumour suppressors have recently been identified as key molecular determinants for de novo nucleotide synthesis that contribute to the maintenance of homeostasis and the proliferation of cancer cells. Inactivation of tumour suppressors such as TP53 and LKB1 and hyperactivation of the mTOR pathway and of oncogenes such as MYC, RAS, and AKT have been shown to fuel nucleotide synthesis in tumour cells. The molecular mechanisms by which these signaling hubs influence metabolism, especially the metabolic pathways for nucleotide synthesis, continue to emerge.”
“Conversion of a normal body cell into a malignant one is now known to require multiple mutations. Three different types of experimental approaches all converged on this important conclusion: epidemiology of human cancers, analyses of DNA in cells at several stages in the development of cancers in humans and mice, and overexpression of oncogenes in cultured cells and transgenic animals.” The transformation of a normal tissue into a malignant tumour (carcinogenesis) is a multistep process, characterized by the gain or loss of gene expression and epigenetic (non-genetic) changes. Such changes lead to the acquiring of the biological characteristics that result in tumour cells progressing from a benign through various malignant phenotypes.2 According to Montréal researcher Dr Richard Momparler, “Epigenetics is described as a heritable change in gene expression without an alteration in the DNA sequence. Research in the past 10 years indicates that epigenetic changes play an important role in tumourigenesis. The major epigenetic changes that take place during the development of cancer are the aberrant DNA methylation of genes that suppress tumourigenesis and histone modifications of chromatin.”
“Carcinogenesis [must] be considered as a complex micro-evolutionary process, which requires the accumulation of a range of (somatic) genetic mutations. Under selection pressure and through these mutations, cells acquire new characteristics, which provide them with an advantage in growth behaviour and other cellular properties, such as enhanced survival and invasiveness. This process is in most cases drawn out over many years and requires a series of individual steps.”
“Blood cells, which are categorized in either the lymphoid or the myeloid lineage, are generated from hematopoietic stem cells (HSCs). Lymphoid lineage cells include T, B, and natural killer (NK) cells, while megakaryocytes and erythrocytes (MegE) as well as granulocytes and macrophages (GM) belong to the myeloid lineage. These two lineages are separable at the progenitor level. Common lymphoid progenitors (CLPs) can differentiate into all types of lymphocytes without noticeable myeloid potential under physiological conditions, although some myeloid related genes might be detected in CLPs, depending on the experimental conditions. Similarly, common myeloid progenitors (CMPs) can give rise to all classes of myeloid cells with no or extensively low levels of B-cell potential. Another cell type, dendritic cells (DCs), is not clearly grouped either in lymphoid or myeloid lineage, because DC can…arise from either CLPs or CMPs.”
“TRM are present in many human cancers (NSCLC, ovarian cancer, bladder cancer, endometrial cancer, melanoma, etc.). Overall, they are associated with a good clinical outcome. Interestingly, the impact of TRM on survival was independent of the infiltration of CD8+ T cells.” A “…novel population of memory CD8+ T cells…has been identified based on their phenotype (CD103, CD69) and on their local tissue residency without recirculating in the blood. These cells have been implicated in protective immune response against pathogens in both animal models and humans.”
“Cytokines play an important role in host defense against microorganisms. They orchestrate innate immunity by inducing protective local inflammation and systemic acute phase responses. Cytokines are important in initiating, amplifying, directing, mediating, and regulating adaptive immunity. Unfortunately, they may also direct tissue damage if excessive responses occur or if they are involved in directing and mediating autoimmunity. Under these circumstances, cytokines are potential therapeutic targets.”
“Under physiological conditions, cells receive fate-determining signals from their tissue surroundings, primarily in the form of polypeptide growth factors. Integration of these extracellular signals underlies tissue homeostasis. Although departure from homeostasis and tumour initiation are instigated by oncogenic mutations rather than by growth factors, the latter are the major regulators of all subsequent steps of tumour progression, namely clonal expansion, invasion across tissue barriers, angiogenesis, and colonization of distant niches”
“Excessive cell proliferation is a feature of most cancers. Limited availability of growth factors or nutrients, contact inhibition, and other feedback mechanisms ensure that the pathways that regulate proliferation are normally tightly controlled.” “…however, mutations in proto-oncogenes and tumour suppressors or inappropriate synthesis of ligands/receptors can hyperactivate these pathways, leading to activation of the cell cycle machinery.” “The Ras-ERK and PI3K-Akt pathways are important regulators of normal cell proliferation and thus their constitutive hyperactivation can lead to excessive proliferation.”
“A pre-metastatic niche is a microenvironment prepared for the colonization of circulating tumour cells in specific organs.” “The key components of the pre-metastatic niche include tumour-derived secreted factors (TDSFs), extracellular vesicles (EVs), bone marrow-derived cells (BMDCs), suppressive immune cells and host stromal cells.” “It is known that cancer cells communicate with fibroblasts via EVs, and this leads to the progression of metastasis. The fibroblasts in tumours are involved in epithelial-mesenchymal transition (EMT) and chemotherapy resistance by contacting…cancer cells and [with] each other.”
“Primary and metastatic tumours are complex ecosystems composed of neoplastic cells, extracellular matrix (ECM), and ‘accessory’ nonneoplastic cells, which include resident mesenchymal support cells, endothelial cells, and infiltrated inflammatory immune cells. Crosstalk between cancer cells and accessory cells fuels and shapes tumour development. During tumour formation, the tissue architecture evolves into a highly specialized microenvironment characterized by a corrupted ECM and chronic inflammation.” In fact now, “It is well known that the crosstalk between cancer cells and stromal components in many tumour types can enhance tumour growth and metastasis.”
“Therapeutic decisions for cancer patients are primarily based on clinical and pathological parameters. In particular, tumour size, histological grade, histotype and immunohistochemical results of prognostic factors play major roles in planning therapeutic strategies (e.g., targeted therapy or chemotherapy). Although this has been a successful approach, many patients relapse and/or eventually develop resistance. Despite the fact that vast technological improvements have increased our understanding of human cancers as heterogeneous diseases, current clinicopathological, immunohistochemical and molecular parameters/markers leave significant numbers of patients at risk for over- or under-treatment.”
“Epithelial-mesenchymal transition [EMT] is the process by which epithelial cells lose cell-cell junctions and baso-apical polarity and acquire plasticity, mobility, invasive capacity, stem-like characteristics, and resistance to apoptosis. This cell biology program is active in embryology, wound healing, and pathologically in cancer metastasis, and along with the mechanical and cellular components of the tumour microenvironment, provides critical impetus for epithelial malignancies to acquire metastatic capability.”
“Polarity of the epithelium serves as a barrier for tumour formation in epithelial tissue by maintaining tissue organization and 3D architecture. The loss of cell polarity and tissue organization is a hallmark of carcinoma. Invasion and metastasis are the major cause of cancer related morbidity and mortality (Hanahan and Weinberg 2011) and appear to be dependent on EMT. EMT is an important hallmark of carcinoma, (Royer and Lu 2011) where migrating tumour cells recapitulate developmental process of EMT by altering the epithelium structure, disrupting the basal lamina and invading the underlying tissues. Loss of integrity of tight and adherence junctions and loss of polarity are crucial features of EMT.” “…cell–cell contact is maintained and regulated by several polarity proteins. These polarity proteins are often targeted by EMT inducers, leading to their altered function, ultimately facilitating cell migration (Martin-Belmonte and Perez-Moreno 2012).”
![]()
